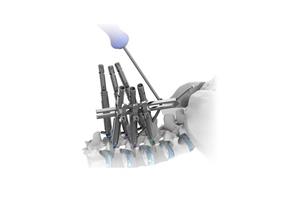
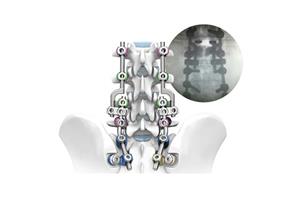
Product Feature
- Manufactured with Mg-Ca Alloy
- Complete absorption ranges from 6 months to 18 months depending on applied indications
- Promotes normal headling process
- No stree shielding
- Radiopaque
- MR compatible
- Proven Biocompatibility (ISO 10993)
- Size OD: Ø 2.7, Ø 3.5, L: 10mm ~ 40mm
INDICATIONS FOR USE
- Carpal, metacarpal and small hand bone
- Tarsal and metatarsals
- Phalanges
- Intra-articular fractures
- Ankle
- Proximal and distal humerus
- Proximal and distal radius
- Proximal and distal ulna
- Osteochondral fixation and fractures
- Osteochondritis dissecans
- Oblique fractures of the fibula
- Reconstructive surgeries of the foot
- Malleolar fixation