Performance

Reasonable price
A positive airway pressure device is known as the most effective among non-invasive treatments. Although some hospitals are already using them, there has been concern regarding its high price in the domestic market. MEK-ICS Co., Ltd.'s M3 is the first positive airway pressure device developed in Korea. We understand patients' concerns and try to decrease their economic burden by offering a more reasonable price.
Reliability
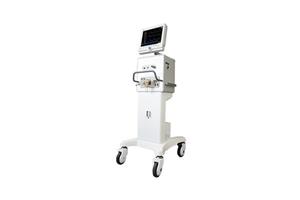
1. The first artificial respirator and positive airway pressure device developed in Korea.
Holding the original technology in the respiration treatment field, MEK-ICS Co., Ltd. has developed the only artificial respirator in Korea. It is exporting products to 45 countries worldwide and leading the respiration treatment in Korea. Moreover, it is a fast-growing company with technology that has the largest market share for patient monitors (monitoring biometric data) in Korea. Based on this technology and after three years of R&D, we have developed the first positive airway pressure device in Korea, the M3.
2. MEK-ICS Co., Ltd. holds the core technology in the respiration treatment field
MEK-ICS Co., Ltd.'s artificial respirator is not just a device that maintains life by adjusting inhalation and exhalation. It minimizes lung damage for patients who do not have adequate self-breathing. It also helps the respiration and recovery of the patient.
The role of an artificial respirator is to insert air during inhalation and let air out during exhalation. The positive airway pressure device must continue inserting air with a certain amount of pressure during the exhalation period. Although there is a difference in the system that adjusts the respiration, there is no significant difference in terms of the technology of respiration treatment. Its core technology for respiration treatment has been applied to the positive airway pressure device including the M3.
At the same time, the technology of patient monitors has been adopted, such as the biological signal measurement module and pulse oximeter, to allow the patient to breathe comfortably.

At the same time, the technology of patient monitors has been adopted, such as the biological signal measurement module and pulse oximeter, to allow the patient to breathe comfortably.
Safety
Compared to other world-class positive airway pressure devices, its function and performance are equal or even better.
During the clinical trial conducted by Samsung Seoul Hospital, M3 and another leading positive airway pressure device were used for patients with obstructive sleep apnea. As a result, M3 showed equal efficacy and safety in improving the sleep apnea and quality of lives of the patients compared to the leading positive airway pressure device.
Also, it is a safe product that passed the performance and safety test by the Ministry of Food and Drug Safety and received approval to manufacture.
*Source: From the result report of the clinical trial by Samsung Seoul Hospital
n improving the sleep apnea and quality of life of patients with obstructive sleep apnea, the positive airway pressure device of MEK-ICS Co., Ltd. has been estimated to show equal efficacy and safety compared to the positive airway pressure device of "R" company.